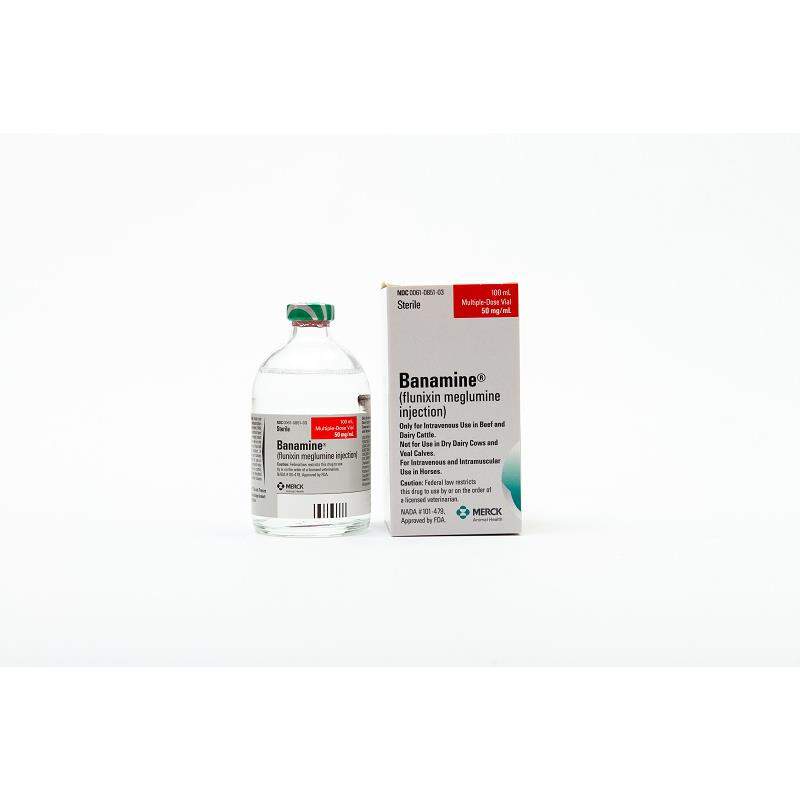
Click on image to open expanded view
Item No.
10092-M
Product Description
Banamine Injectable is a sterile flunixin meglumine solution that is used to treat inflammation and discomfort in horses suffering from musculoskeletal diseases and colic. In the USA, it is also permitted for use in cattle.
Who is Banamine Injectable for?
Horses and Cattle
Why use Banamine Injectable?
Banamine Injectable is used to treat musculoskeletal diseases by reducing inflammation. It is an excellent remedy for horses with lameness and discomfort. It provides cattle and horses with immediate comfort.
How does Banamine Injectable work?
Flunixin meglumine, the main component in Banamine Injectable Solution, is an antipyretic non-steroidal anti-inflammatory medication (NSAID) that reduces inflammation and discomfort in cattle and horses.
Manufacturer:
Merck Animal Health.
Active Ingredients(s):
FLUNIXIN MEGLUMINE
How is Banamine Injectable sold?
100ml 250ml
What are the side effects of Banamine Injectable?
In rare cases, horses have experienced a local reaction following intramuscular injection - especially those in the neck. These may include: Swelling Sweating Induration Stiffness In rare cases, horses and cattle have experienced experienced anaphylactic-like responses following intravenous injections. These have proven fatal in some cases. In rare cases, infections have been reported following intramuscular injections of flunixin meglumine. These include fatal clostridial infections.
What special precautions are there?
Precautions for Use in Horses: Drug compatibility should be regularly checked in patients who require supplementary therapy. There haven't been any studies done to eliminate out interactions with other drugs taken at the same time. This product's impact on pregnancy is unclear. Precautions for Use in Cattle: This product should not be given to a breeding bull since reproductive effects have not been evaluated. Both estrus and parturition are known to be affected by NSAIDs. Tocolytic drugs like NSAIDs might cause a delay in parturition. Estrus onset might be slowed if given during the prostaglandin phase of the estrus cycle. NSAIDs can interfere with the ejection of the fetal membrane or induce uterine involution if given soon after delivery. The cow should be closely checked for retained placenta if this product is used within 24 hours of parturition.
What to do if overdose?
Reach out to the nearest animal clinic for guidance. You may also contact Merck Animal Health Pharmacovigilance: Livestock Technical Services, 800-211-3573
How can I store Banamine Injectable?
Store between 36°(2°C) -86°F (30°C).
Helpful Tips:
Before you use this product, read the package insert and directions carefully.
Overview
The suggested daily dosage for treating musculoskeletal diseases in horses is 0.5mg per pound of body weight (1mL per 100 pounds). For a maximum of 5 days, administer intravenously or intramuscularly. Within 2 hours, activity begins, and maximal response occurs between 12 and 16 hours. The action lasts between 24 and 36 hours. The suggested dose for horse colic pain treatment is 0.5 mg per pound of body weight given intravenously for immediate relief. In most cases, pain should be alleviated within 15 minutes. When symptoms persist, treatment can be repeated. In a clinical study, around 10% of horses required one or two further treatments. To address the etiology of colic, concurrent treatment should be employed. For management of bovine respiratory illness, pyrexia associated with endotoxemia, and inflammation in endotoxemia in cattle, the suggested dose is 0.5mg to 1mg per pound of body weight (1mL to 2mL per 100 pounds). Via slow, once-daily intravenously injection, or in two split half-doses injected 12 hours apart for three days in a row. A total dosage of 1mg per pound of body weight should never be exceeded. Do not give the medication too quickly. 1mg per pound of body weight (2mL per 100 pounds) injected intravenously is the recommended dosage for treating bovine mastitis.
Main Ingredients
Each milliliter of BANAMINE Injectable Solution contains flunixin meglumine equivalent to 50 mg flunixin. 0.1 mg edetate disodium 2.5 mg sodium formaldehyde sulfoxylate 4.0 mg diethanolamine 207.2 mg propylene glycol 5.0 mg phenol as preservative, hydrochloric acid, water for injection qs